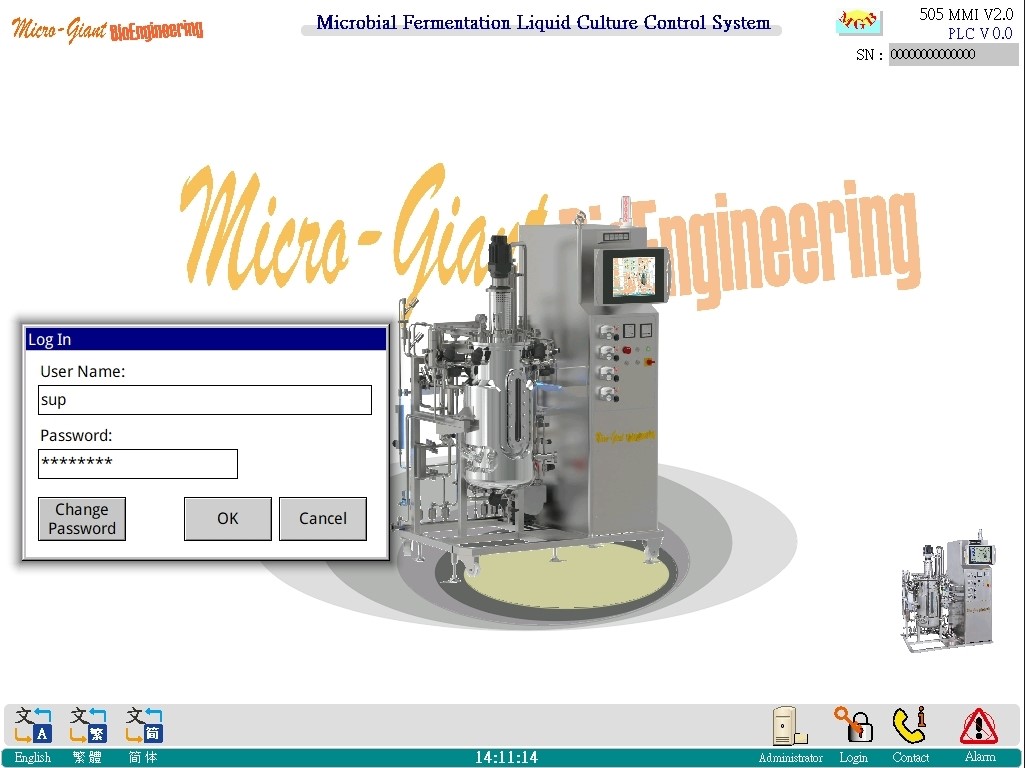
Login screen:
◎ Comply with FDA 21 CFR (data record saving, authority management division, audit trail, data log collection)
◎Category and record user permissions when logging in. Each person has his or her unique user name and password.
◎ Multiple lanaguage interfaces (can be customized) for selection or online changes.
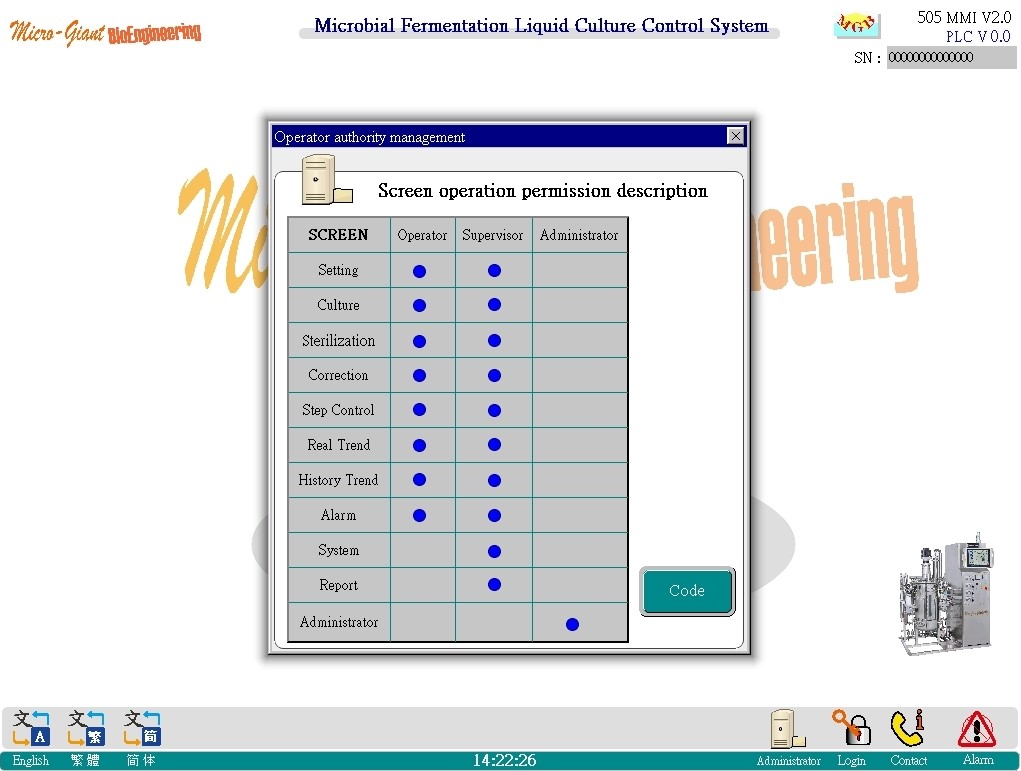
Administrator and password screen:
◎ Chart description of the permission differentiation of each group: operator/ supervisior/administrator.
◎ The administrator function allows users to add and delete users and provide the original password. After the user logs in with the original password, the user can immediately change the new password.
◎ Only authorized personnel can do appropriate things.
◎ The maximum number of users can be 32.

Culture screen:
◎ Graphical control interface for multiple culture methods: microbial, cell, absolute anaerobic, liquid, solid, continuous, semi-continuous, batch... culture.
◎ Dynamic real-time display of agitation or foaming icons and fluid direction, automatic or component icons, manual/automatic turn on of segmented display.
◎ In addition to the automatic control mode, it has an online manual switch. It can be forced to open and close under safety conditions.
◎ If the screen does not receive new touches within the set time, it will "LOCK" by itself, or it can be locked directly to avoid accidental presses.
◎ "HOST" key can be turned on (Host On) to deliver remote SCADA software for remote control.
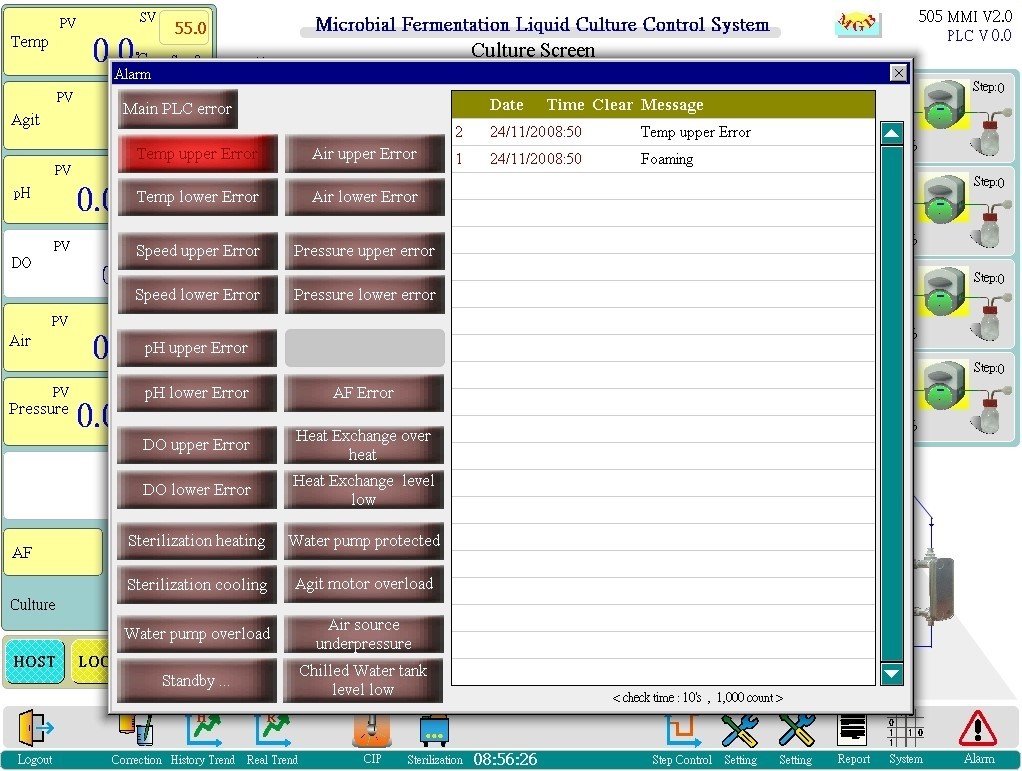
Alarm screen:
◎ Can record at least 1,000 alarm messages. Record message content, occurrence and lift time. If exceeded, the last 1,000 messages will be retained and saved.
◎ Real-time alarm messages will light up on the left screen and be recorded and saved.
◎ When PLC is abnormal, the abnormal code will be displayed and appear.
◎ The alarm sound can be turned on or off, and the alarm light (on the screen and the control box) will flash when the alarm occurs.

Report screen:
◎ The operation record can record at least 1,200 transactions and online inquiries. The record content includes the operator, time, and all operation content.
◎ It can be used as an audit trail. It can also be saved as a PDF file to an external device through the USB interface of the control box. (Alarms can be queried online in real time on the alarm screen.)
◎ Real-time trend data log records are saved in PDF or CSV format to external device through the USB interface of the control box.
◎ Data collection is saved in PDF file to avoid data tampering and complies with FDA 21 CFR PART 11.

Data log screen:
◎ Real-time trend and data log can record up to 35,000 pieces of data. Each piece of data contains temperature, agit, pH, DO, air, ORP, load cell, tank pressure....current values and date and time.
◎ Each sampling time can be set from 3~3,600 seconds. If one record is recorded in 60 seconds, it can be recorded for 24.3 days, if the time exceeds, the last 35,000 records will be retained and saved.
◎ Can download historical trends (up to 3 sets) for online comparison and analysis.
◎ When foaming occurs, the occurrence and lift times can be recorded, which is helpful for understanding growth characteristics.
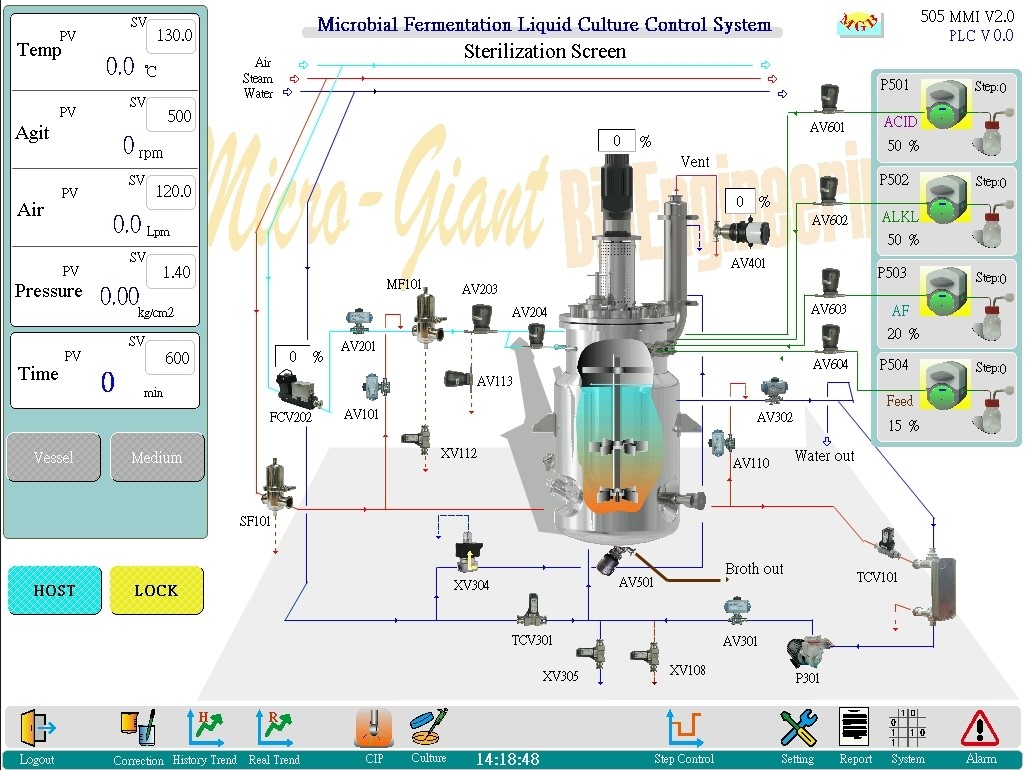
Sterilization screen:
◎ From this screen to proceed vessel sterilization and medium sterilization, the status of all valves during the process can be undertood from the pipeline diagram on the screen.
◎ The purpose of vessel sterilization is to preparatory sterilize the sterile pipelines and vessel before performing medium sterilization.
The purpose of medium sterilization is to sterilize the culture medium.
◎ The current sterilization stage in progress will be displayed in the lower left corner of the screen.
e.g. Heating 1 → Heating 2→ Sterilization→ Cooling 1→ Cooling 2→ Stand by.
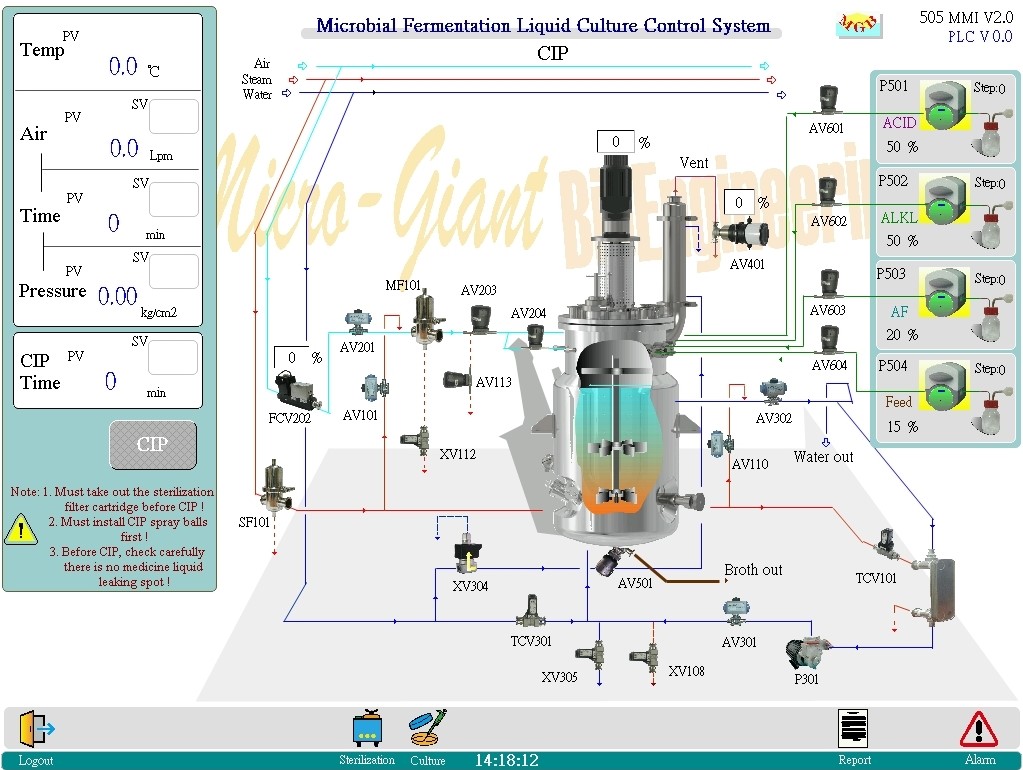
CIP screen:
◎ From this screen to proceed CIP, the status of all valves during the process can be understood from the pipeline diagram on the screen.
